Getty Images
Clinical trials are a critical part of the research process for new medicines and vaccines. The information gleaned from these studied is essential to developing new methods for preventing, treating and in some cases curing disease.
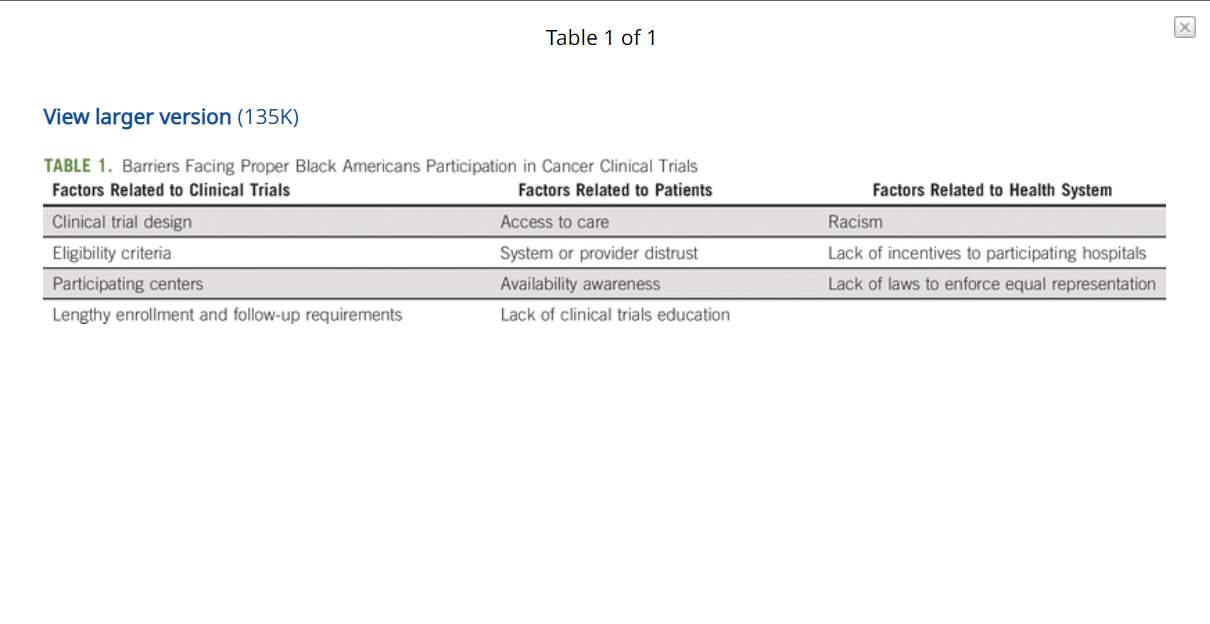
Clinical trials have also become another intersection for race and medicine, with people of color woefully underrepresented in important research to improve health outcomes for patients with particular diseases or medical maladies. These studies primarily enroll white, male patients, who makeup the vast majority of participants in clinical trials.
The Participatory Action for Access to Clinical Trials project at the University of Michigan is a community-based participatory research study working to understand Black and African American individuals’ low levels of participation in clinical research trials, examine the determinants influencing current motivation and ability to participate, and develop and pilot a strategy to increase minority enrollment in clinical trials.
PAACT notes that people of color make up about 39 percent of the U.S. population, but Black and Hispanic Americans represent only about 7 percent of patients participating in clinical trials. And while Black Americans comprise 13 percent of the U.S. population, yet only 5 percent of clinical trial participants are Black.
The dangers in this extreme lack of representation in investigating the symptoms and causes of disease and identifying solutions are glaring and contribute to the grosse disparities in diagnosis and treatment, and ultimately a diminished quality of life and health standards in Black and Brown communities.
Reasons for lack of participation …
But why aren’t Back people participating. Let’s start with the obvious – mistrust of the medical profession and medical science are a given. The more well known and egregious abuses of Black people in medical research – The Tuskegee Experiment and the case of Henrietta Lacks – along with numerous other less well-known transgressions, abuses and negligence are common complaints from Black and Brown patients continue to erode levels of confidence in doctors and scientists.
Why minority participation in clinical trials is crucial for better health outcomes …
Medical experts, scientists and researchers agree that for trials to effectively investigate and track factors contributing to disease and health disorders, they have to be more reflective of the patient population. If White men and women makeup the overwhelming majority of participants in these studies, consequently drugs and treatments developed are based on responses in that population and primarily benefit patients of that or similar ethnicity. The efficiency of treatments for those of other ethnicities remains questionable.
Finally some, trials can only be conducted with the participation of Black patients and volunteers, as is the case with sickle cell anemia, rising maternal mortality rates, kidney failure, diabetes, and the skyrocketing rates of cancer in Black men which is 70 percent higher than that of their white counterparts.
Solutions to the problem …
Companies rarely face public scrutiny, don’t need a representative trial to secure the approval of the U.S. Food and Drug Administration (FDA), and believe that recruiting representative patients adds time and expense. Conducting diverse trials has not been mandated and has not been a priority.
To circumvent that blatant lack of interest in involving minority patients in these treatment and cure seeking trials the Harvard Business Review, PAACT and the National Library of Public Medicine are putting forth several recommended courses of action to address the problem.
Take it to the streets – One of the biggest barriers to participating in a clinical trial is getting there. The typical trial requires patients to repeatedly travel to a central site — such as a university hospital — for assessments, administration of therapies, tests to monitor results, and medications to take at home.
“Partnerships with local organizations – groups who know their people best — can help. We’re also running our trials in more areas. Last year, about half of our clinical trial locations were placed in areas where underrepresented groups live to meet them where they are,” explains Adrelia Allen, senior director, Clinical Trial Patient Diversity, Merck Research
Another promising solution involves the use of patient navigators to help enhance clinical trial recruitment, enrollment, and retention. “We’re training more people to help volunteer participants through the clinical trial process called Patient Navigators,” adds Allen. “Patient Navigators can work with patients and medical staff as a go-between. We’re also looking at how to run trials so that some aspects may be easier for volunteer participants, like using telemedicine.”
Change trial criteria – Efforts to increase diversity must begin long before a trial gets underway — that is, with the design of the study itself, according to research leaders. “So even if you have a disease and want to participate in a study, the exclusion criteria will kick you out,” explains patient advocate Quita Beeler Highsmith, formerly of Gentech.
She suggests that study designers can broaden the eligibility criteria for trials and reduce the criteria that exclude people. She observes that many study authors use exclusion criteria — typically for conditions such as a certain body mass index or white blood cell count.
To that end, The National Institutes of Health now requires grant applications for trials to include plans for recruiting women and members of minority groups. The Food and Drug Administration issued guidance in November 2020 that includes broadening eligibility criteria, avoiding “unnecessary” exclusions, and improving recruitment so that participants “will better reflect the population most likely to use the drug.

Challenge assumptions
The commonly accepted notion that some groups are significantly underrepresented in trials because they don’t want to participate is wrong and creates a self-fulfilling prophecy, as researchers summarily write off those populations and potential participants.
“When we’ve studied this assumption by asking people from underrepresented groups why they don’t participate in trials, they say, ‘I was never approached, ’” notes notes Monica Baskin, PhD, associate director for community outreach and engagement at the University of Alabama School of Medicine.
The bottomline – clinical trials are critical to identifying, understanding and developing remedies for what ails us, all of us. Better representation of Black and Brown patients is a must if these trials are to have any validity for treating diseases that affect all and every American, regardless of ethnicity.
“We need to do a better job engaging people in underrepresented communities and help them understand what participation in a clinical trial involves,” concludes Allen.